Abstract
Purpose
Advanced maternal age (AMA) is a known risk factor for pregnancy-related venous thromboembolism. However, it is unclear if underlying differences exist in the maternal coagulation profiles of AMA vs non-AMA women. The aim of this prospective observational study was to compare peripartum thromboelastography parameters of AMA and non-AMA women undergoing elective Cesarean delivery (CD).
Methods
We compared the peripartum thromboelastographic profiles of healthy AMA women (age > 35 yr) and non-AMA women (age ≤ 35 yr) undergoing elective CD under neuraxial anesthesia. Blood samples were drawn prior to CD and at 24 hr and 72 hr post-CD. At each time point, we assessed thromboelastographic and other standard laboratory coagulation indices. We used a linear mixed-effects regression model (SAS® PROC MIXED) to assess between-group differences for individual thromboelastographic and laboratory coagulation parameters.
Results
The median [interquartile range] ages of women were 38 [37-41] yr and 29 [25-34] yr in the AMA and non-AMA groups, respectively (P < 0.001). We observed no statistically significant effect of study group on any thromboelastographic or laboratory coagulation parameters. No statistically significant correlations were found between any thromboelastographic parameter and maternal age. Peripartum thromboelastography and coagulation profiles of healthy AMA and healthy non-AMA women up to 72 hr post-CD were also similar.
Conclusion
These data suggest that maternal thromboelastographic profiles of healthy AMA and non-AMA women undergoing elective CD are similar. The study was registered at ClinicalTrials.gov (identifier: NCT01416454).
Résumé
Objectif
L’âge maternel avancé (AMA) est un facteur de risque connu d’événements thromboemboliques veineux liés à la grossesse. Néanmoins, nous ne savons pas avec certitude s’il existe des différences sous-jacentes dans les profils de coagulation maternels chez les femmes AMA par rapport aux autres femmes non AMA. Le but de cette étude observationnelle prospective était de comparer les paramètres de thrombo-élastographie du péripartum chez les mères AMA et non AMA devant accoucher par césarienne programmée.
Méthodes
Nous avons comparé les profils thrombo-élastographiques du péripartum chez des femmes AMA en bonne santé (âge > 35 ans) et des femmes non AMA (âge ≤ 35 ans) subissant une césarienne programmée sous anesthésie neuraxiale. Des échantillons de sang ont été prélevés avant la césarienne, puis 24 h et 72 h après l’intervention. À chaque moment, nous avons évalué les index thrombo-élastographiques ainsi que les autres paramètres de laboratoire standard de la coagulation. Nous avons utilisé un modèle de régression linéaire d’effets mélangés (SAS® PROC MIXED) pour évaluer les différences entre les groupes concernant les résultats individuels des paramètres thrombo-élastographiques et de coagulation.
Résultats
Les âges médians (intervalle interquartile) des femmes étaient de 38 (37-41) ans et 29 (25-34) ans pour, respectivement, les groupes AMA et non AMA (P < 0,001). Nous n’avons observé aucun effet statistiquement significatif de groupe d’étude sur les paramètres thrombo-élastographiques ou sur les paramètres de laboratoire de la coagulation. Aucune corrélation statistiquement significative n’a été trouvée entre des paramètres thrombo-élastographiques et l’âge maternel. Les profils de thrombo-élastographie et de coagulation du péripartum de femmes AMA et non AMA en bonne santé ont été également similaires jusqu’à 72 heures après la césarienne.
Conclusion
Ces données suggèrent que les profils thrombo-élastographiques de femmes AMA et non AMA en bonne santé subissant une césarienne programmée sont semblables. Cette étude a été enregistrée sur le site ClinicalTrials.gov sous le numéro: NCT01416454.
Similar content being viewed by others
Pregnancy is associated with a hypercoagulable state which teleologically offers women protection from excess blood loss at the time of parturition.1 Pregnant women are at increased risk for venous thromboembolism (VTE), a leading cause of pregnancy-related maternal death in developed countries.2 , 3 In several population-wide studies, advanced maternal age (AMA) has been associated with an increased risk of pregnancy-related VTE;4 - 6 however, the pathophysiological basis that explains why AMA women are at increased risk of VTE compared with non-AMA women has not been adequately investigated.
Point-of-care technologies, such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM), allow examination of the viscoelastic properties of clot formation.7 These technologies have been used to verify the presence of hypercoagulability in pregnant and postpartum women8 - 10 and have also been used to detect a positive association between blood hypercoagulability and age in non-obstetric patients.11 , 12 Furthermore, previous non-obstetric studies have reported that hypercoagulable states detected by TEG are associated with an increased risk of thrombotic complications, including myocardial infarction and thromboembolic events.13 - 15 Nevertheless, these technologies have not been used to investigate whether differences exist in the maternal coagulation profile of AMA vs non-AMA women during the peripartum period.
Mode of delivery may also influence the risk of VTE in the postpartum period. Compared with vaginal delivery, the risk of postpartum VTE is increased two to fourfold in women undergoing Cesarean delivery (CD).5 , 16 , 17 Therefore, examination of the maternal thromboelastographic profiles of AMA women and non-AMA women undergoing CD may provide important insight about endogenous changes in the coagulation profiles of women at low risk and high risk for VTE. These data may provide an important first step in determining why AMA women are at increased risk of VTE. Research in this field is of clinical importance as many women in developed countries desire to have children after 35 yr of age.18
We hypothesized that AMA women undergoing elective CD have a greater degree of hypercoagulability compared with non-AMA women. To investigate our hypothesis, we performed a prospective study to compare the peripartum TEG profiles of AMA and non-AMA women undergoing elective CD under neuraxial anesthesia.
Methods
After gaining approval from Stanford University Institutional Review Board (IRB approval number: 19878; October 2010) and written informed consent, participants were enrolled in this prospective study at Lucile Packard Children’s Hospital, Stanford, California. Patients presenting for elective CD under neuraxial anesthesia were eligible for enrolment. Due to the low number of healthy AMA women with uncomplicated pregnancies admitted for elective CD, we commenced the study in November 2010 and completed the study in August 2012. Inclusion criteria were: American Society of Anesthesiologists physical status I or II, patients with uncomplicated singleton pregnancies, and gestational age of 37-42 weeks. Exclusion criteria were: non-elective CD; age < 18 yr; significant medical or obstetric comorbidity (such as essential hypertension, liver disease, diabetes mellitus, preeclampsia); inherited or acquired coagulation disorders; thrombocytopenia (platelet count < 100 × 109·L−1); patients receiving acetylsalicylic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), or any anticoagulant; patients in early or active labour; documented uterine or placental abnormalities; pregnancies by in vitro fertilization (IVF); and multiple gestation. At our institution, NSAIDs have been used for routine post-Cesarean analgesia orders. For this study, post-Cesarean NSAIDs were withheld until after the final study measurements were taken.
We defined our two study groups using the following maternal age cut-off points: AMA as maternal age > 35 yr and non-AMA as maternal age ≤ 35 yr; we used the maternal age on the date of the CD. In order to limit selection bias, we used an allocation concealment method to determine the sequence of patient recruitment. Qualified AMA and non-AMA women were recruited in a randomized order to minimize any selection and temporal biases. A randomization sequence (block randomization with a 1:1 ratio for study groups) was generated to ensure that there was an adequate balance of women over the period of study recruitment. Patient enrolment was overseen by study investigators (M.C.G., G.H., A.B.) who were not members of the primary anesthesia or obstetric team. All women underwent elective CD under the supervision of an experienced attending (consultant-level) obstetrician and anesthesiologist who were not involved in the study.
In keeping with standard institutional practice at the time of the study, all patients received intravenous fluid preloading with 6% hetastarch 500 mL in normal saline (Hespan®; B. Braun Medical Inc., Irvine, CA, USA) before neuraxial anesthesia was performed. The colloid preload was infused within 30 min of neuraxial anesthesia (spinal or combined spinal-epidural anesthesia). A fluid preload of 6% hetastarch 500 mL has also been shown to have a minimal effect on the maternal thromboelastographic profile when compared with crystalloid 1,500 mL.19
The neuraxial block was placed at the L3-4 interspace. All patients received intrathecal 0.75% hyperbaric bupivacaine 1.6 mL, fentanyl 10 μg, and morphine 200 μg. After neuraxial block placement, the patients were placed in the supine position with left lateral uterine displacement. After confirming adequate surgical anesthesia (bilateral T5 sensory level to pinprick), the obstetrician commenced surgery. The anesthesia team was asked to restrict the total crystalloid volume to < 2 L for the intraoperative period. After delivery of the fetus, all patients received a 2 U bolus of oxytocin iv followed by an infusion of oxytocin commenced initially at 3.75 U·hr−1. The supervising anesthesiologist altered the oxytocin infusion rate as necessary based on the obstetrician’s assessment of the adequacy of uterine tone. Total estimated blood loss (EBL) was calculated after completion of surgery. Using a previously described approach,20 the total EBL was estimated as the sum total of the volume of blood in the suction chamber, the weight of blood on blood-soaked surgical swabs using electronic scales, and blood loss around the surgical field. We also collected data for the duration of surgery, total dose of intravenous oxytocin administered, and the total volume of infused intravenous fluids (crystalloid and colloid).
Blood sampling and assays
We performed blood sampling for TEG analyses and laboratory analyses at three time points: before surgery (baseline values), 24 hr post-CD, and 72 hr post-CD. Prior to surgery, an 18G peripheral intravenous cannula was inserted into a forearm vein under minimal stasis with a tourniquet. The first 4 mL of withdrawn blood were discarded in a syringe to avoid tissue contamination, and a second syringe was used to collect venous blood for TEG and laboratory analysis. For the post-CD blood samples, a 19G butterfly needle was used to obtain blood from a peripheral vein (non-intravenous fluid arm). At each study time point, venous blood was collected for the following hematological and coagulation indices: hemoglobin level, platelet (PLT) count, prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen levels. Normal non-pregnant values for these variables in our laboratory are PLT 150 − 400 × 109·L−1, PT 11.5-14.2 sec, APTT 23.9-36.7 sec, international normalized ratio 0.9-1.2, and fibrinogen 236-389 mg·dL−1.
Thromboelastography assays were performed using a Thrombelastograph® 5000 Analyzer (Haemonetics® Corp., Braintree, MA, USA). All TEG analyses were commenced within four minutes of venous blood collection. We performed each TEG analysis using native whole blood and disposable cups and pins. Initially, we pipetted 1 mL of whole blood into a vial containing the intrinsic pathway activator, kaolin (40 μL in 0.85% saline), followed by mixing by inversion. For each assay, kaolin-activated whole blood 360 μL was pipetted into a plastic cup and positioned in the pre-warmed (37°C) Thrombelastograph Analyzer.
We recorded the following TEG parameters: r time (minutes), k time (minutes), α angle (degrees), MA (mm), G (dynes·sec−1), and CL30 (%). The r (reaction) time represents the rate of initial fibrin formation and is the time taken from the start of the TEG recording until initial fibrin formation occurs (at the point when the amplitude = 2 mm). The k time represents the dynamics of clot growth and is the time measured from the end of the r time until the amplitude reaches 20 mm. The α angle represents the rate of fibrin build-up and cross-linking and is the angle of the slope of the TEG trace from the end of r to the end of k. The MA represents the maximum amplitude (strength) of the fibrin clot. The G represents the shear elastic modulus strength and is calculated from the progressive increase in amplitude as the TEG trace develops. The G is inclusive of both enzymatic and platelet contributions to overall clot strength.21 The CL30 refers to the percentage of clot lysis at 30 min after MA. We also recorded dynamic properties of clot formation which represent the kinetic properties of thrombus formation. These parameters are calculated by transforming TEG data into a velocity curve (first derivative of clot strength vs time, V curve). Thromboelastography parameters of thrombus generation recorded in our study were: total thrombus generation (TTG), maximal rate of thrombus generation (MRTG), and time to maximal rate of thrombus generation (TMRTG). The TTG is derived from the total positive area under the velocity curve during clot growth (dynes·cm−2). The MRTG is the maximum rate of clot strength development and is the first derivative of the velocity of increase in clot strength (dynes·cm−2·sec−1). The TMRTG is the time to initiation of clot formation (lag time) plus the time taken for clot development to reach MRTG (minutes).
Statistical analysis
Based on work by Ng et al., who observed associations between aging and r time, k time, α angle, and MA,12 and Boyce et al., who observed that r time displays the greatest degree of change up to four hours post-elective CD,22 we selected r time for calculating sample size in our study. To estimate differences in r times between study groups, the sample size was estimated from prior studies that reported the incidence and risk of VTE in pregnancy among AMA and non-AMA women5 , 6 and from TEG data from our previous study investigating changes in the maternal coagulation profile before and after CD.23 Based on these data, we predicted that 22 patients per group would be necessary to observe a 25% decrease in r time in AMA women compared with non-AMA women [baseline mean (SD) r time = 7.6 (2.2) min for non-AMA women], with an alpha of 0.05 and power of 80%. To account for protocol violations or technical difficulties with TEG analyses, we aimed to enroll 23 patients per group for our study. Data were assessed for normality using normality plots and Kolmogorov-Smirnov tests. Data are presented as mean (SD) or median [IQR]. The maternal demographic, obstetric, and perioperative continuous data were analyzed using the unpaired Student’s t test and Mann-Whitney U test, where appropriate. We used a linear mixed-effects regression model (SAS PROC MIXED) to assess between-group differences for individual TEG parameters. For each model, we treated each individual TEG parameter as the response variable, with time as a repeated effect and study group as a fixed effect, and accounted for within-subject correlation for repeated measurements in each patient. The degrees of freedom for model inference were calculated using the Kenward-Roger method. Using the relevant linear mixed-effects model, we calculated the mean between-group difference and 95% confidence intervals for each TEG parameter at each time point. The 95% confidence intervals were calculated from the standard error estimate of the least-square mean difference between study groups. For each study group, we also assessed differences for each TEG parameter between study time points. For our secondary analyses, we performed linear regression to assess the association between maternal age and individual TEG and laboratory indices (baseline and post-CD). All analyses were performed using SAS® 9.3 (SAS Institute, Cary, NC, USA). All recorded P values are two sided.
Results
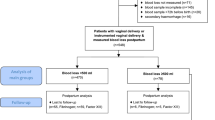
Seventy-three patients were screened and 46 patients were recruited (Figure). The median [IQR] ages of women were 38 [37-41] yr and 29 [25-34] yr in the AMA and non-AMA groups, respectively (Table 1). Other maternal demographic and obstetric characteristics were similar in both groups (Table 1). In the AMA group, we were unable to collect blood samples for TEG analysis at 72 hr on three patients (one patient withdrew consent for venipuncture for TEG analysis, and two women were discharged before 72 hr). In the non-AMA group, we were unable to collect blood samples for TEG analysis on six patients (one was discharged before 72 hr, four patients were commenced on NSAIDs, and one patient was prescribed enoxaparin at 72 hr). Perioperative data, including duration of surgery, total EBL, total dose of intravenous oxytocin administered, and total infused crystalloid and colloid volumes, were also similar in both groups (Table 2). Laboratory hematological and coagulation indices are presented in Table 3. Based on mixed-effects models, we observed no statistically significant effect of study group on any hematological or coagulation value.
Thromboelastography data for the study groups are presented in Table 4. Based on the results of individual mixed-effects models, we observed no statistically significant effect of study group on any standard or derived TEG parameter; however, we did observe statistically significant within-group differences for G and CL30 over time. For both study groups, we observed significant differences between baseline vs 24 hr post-CD values for G (P < 0.05, respectively) and between 24 hr and 72 hr post-CD values for G (P < 0.05, respectively) (Table 4). In the non-AMA group, we observed a significant difference between baseline and 24 hr post-CD values for CL30 (P < 0.05). Data for the between-group differences for individual TEG parameters at each measured time point are presented in Table 5. We observed no significant directional change towards hypo- or hypercoagulability when we compared the differences for individual TEG parameters between non-AMA women and AMA women.
In our secondary analysis, no statistically significant correlations were found between any TEG parameter at baseline, 24 hr, or 72 hr post-CD and maternal age (data not presented).
Discussion
The findings of our study suggest that healthy AMA women may have pre- and post-CD thromboelastographic and coagulation profiles similar to those of healthy non-AMA women at term gestation. In addition, our results suggest that maternal age may not influence individual thromboelastographic or laboratory hematological parameters of healthy women before or up to 72 hr post-CD. Although this study was exploratory, we limited the influence of other medical/peripartum factors on the coagulation profile by selecting only healthy AMA and non-AMA women for pre-labour elective CD. By setting these inclusion criteria, we were able to determine with more certainty whether thromboelastographic indices differ between AMA and non-AMA women.
Although the hormonal-related decrease in venous capacitance and venous outflow are presumed to be responsible for the increased risk of thrombosis in pregnant patients,24 it has been unclear whether age-related changes in the maternal coagulation profile also influence the risk of postpartum VTE. In several population-wide studies, women aged ≥ 35 yr were observed to be at increased risk of pregnancy-related VTE compared with women aged < 35 yr.4 – 6 Advanced maternal age women 35-44 yr of age may also be at increased risk of postpartum VTE compared with women 15-24 yr of age.25 Nevertheless, data from other studies suggest that this association should be questioned. In a Danish cohort study investigating pregnancy-associated VTE, maternal age was not associated with an increased risk of VTE, and no interaction was reported between maternal age and gestational age.26 Furthermore, in a recent prospective study of AMA women with uncomplicated pregnancies undergoing elective CD with either post-CD thromboprophylaxis for seven days or no thromboprophylaxis, no thromboembolic events were diagnosed in either group up to six weeks after delivery.27
Results from prior studies verify that TEG possesses the capability of identifying the hypercoagulability associated with pregnancy. Compared with non-pregnant controls, data from these thromboelastographic studies indicate that pregnant women have shorter r times,8 , 28 - 30 shorter k times,8 , 28 , 30 longer MA and α angles,8 , 28 , 30 and greater coagulation index values.28 , 30 Similar findings were observed in a longitudinal study reporting TEG variables during and after pregnancy.9 Compared with TEG variables eight weeks postpartum, the mean reductions in r time and k time during pregnancy varied from 23-26% to 18-35%, respectively, and the mean increases in MA and α angle during pregnancy varied from 12-20% to 6-8%, respectively.9 These findings are consistent with the increase in coagulation factors and platelet reactivity and the decrease in endogenous anticoagulant activity that accompany pregnancy.1 Furthermore, the baseline TEG values in our study groups are similar to those reported by Macafee et al. who assessed normal TEG values in a healthy cohort undergoing elective CD under spinal anesthesia; the mean (SD) age of women in this study was 34 (4.1) yr.10
We observed evidence of time-dependent changes in the maternal coagulation profiles of AMA and non-AMA women. In both groups, statistically significant, yet modest, decreases in G (shear elastic modulus) values were observed at 24 hr post-CD compared with preoperative values. These changes in G may represent a response to a decrease in the concentration of clotting factors, platelets, and fibrinogen secondary to blood loss at delivery and hemodilution from intravenous fluids administered peri- and post-CD. The modest downward trend in CL30 may be due to a slight decrease in fibrinolysis at 24 hr after CD. Between 24 hr and 72 hr post-CD, the G and CL30 values increased modestly. Other studies that have assessed TEG indices in women undergoing elective CD have shown inconsistent results. Macafee et al. assessed TEG profiles in healthy women undergoing elective CD and reported that preoperative TEG indices were similar to postoperative TEG indices measured immediately after CD or four hours after thromboprophylaxis post-CD.10 In contrast, Boyce et al. observed shorter r times and k times and wider alpha angles at three hours post-CD compared with indices recorded prior to surgery.22 Sharma et al. observed higher alpha angle values for postpartum women 12-24 hr post-delivery compared with term pregnant women.8 Nevertheless, postpartum values were not measured in women post-CD, and no other significant differences were observed in other TEG indices in pregnant vs postpartum women. The influence of maternal age on peripartum TEG indices was also not reported in these studies.
From the preoperative period to 72 hr post-CD, we observed only modest within-group changes in individual TEG variables. These findings are consistent with those of other studies indicating that the maternal hypercoagulable profile may persist into the early postpartum period. Within the first postpartum week, factor II, factor VII, factor IX, C-reactive protein, fibrinogen, antithrombin, and markers of thrombin generation can remain at pre-delivery levels or increase after delivery.31 - 33 In keeping with these observations, the incidence of postpartum VTE is highest during the first postpartum week.6 , 34 , 35 It is unclear when peak hypercoagulable changes occur during the early postpartum period or whether the risk of postpartum VTE is influenced to a greater degree by dynamic changes in the maternal coagulation profile.
We acknowledge a number of study limitations. One of the main limitations is that our study may have been underpowered to show a difference in r time between study groups. When we initiated the study, no age-specific data were available on the maternal coagulation profile of term women. For our power analysis, we assumed that any difference in the degree of maternal hypercoagulability would reflect the known risk difference for VTE between AMA vs non-AMA women. Based on previous epidemiologic data, we conservatively estimated a 25% risk difference for early postpartum VTE between AMA and non-AMA women.5 , 6 Based on our results, we performed a post hoc power analysis to determine the sample size needed for a future study. For a two-sided 95% confidence interval for a two-sample normal mean between-group difference in r time at 24 hr, assuming a standard deviation of 2.7, a sample size of 97 patients per group would be needed to obtain a half-width (half the width of the confidence interval) of at most 0.8 with a conditional probability of at least 0.8, given that the interval contains the true mean difference. Given the challenges and time investment needed to identify and recruit healthy AMA and non-AMA patients at a single institution, in our view, a multicentre study would be needed to constitute a sufficient sample size to perform a more complete investigation of the differences in TEG values between AMA and non-AMA women.
It is unclear whether all women who undergo CD are at increased risk of VTE. Previous studies have observed that, compared with vaginal delivery, women who undergo CD have a two to fourfold increased risk of VTE.5 , 16 , 17 It is unclear, however, whether the maternal coagulation profile and the risk of VTE vary according to the timing and indication of CD. In two separate studies, Jacobsen et al. reported that the risk of VTE was higher in women undergoing emergency CD compared with planned CD.17 , 36 Because of the strict inclusion criteria for our study, the external validity of our findings is limited. Thus, it is uncertain whether these findings apply to all AMA women, such as those with a medical or obstetric comorbidity or those with an IVF pregnancy. Women with an IVF pregnancy are at increased risk of VTE and arterial thrombosis as these patients are known to develop enhanced hypercoagulability due to ovarian hyperstimulation syndrome after IVF.37 We defined AMA as maternal age > 35 yr and non-AMA as maternal age ≤ 35 yr. Our definition is slightly different from other studies that describe AMA as age ≥ 35 yr.4 - 6 Nevertheless, the AMA cut-off point is unlikely to have altered our main findings, especially as we did not observe significant correlations between any TEG variable and maternal age at the measured study time points. It is uncertain whether TEG is sufficiently sensitive to detect subtle differences in the degree of maternal hypercoagulability. Although previous non-obstetric studies have used TEG to detect a hypercoagulable state associated with venous thrombosis,13 - 15 future population-wide studies incorporating more sensitive techniques are needed for a full evaluation of the potential associations between thrombin generation and platelet reactivity with postpartum VTE.
In conclusion, in this exploratory study, we did not observe any differences in the TEG profiles of healthy AMA vs non-AMA women before and up to 72 hr post-CD. In addition, we did not observe any significant associations between maternal age and individual TEG or laboratory coagulation indices. Future population-wide studies are needed to investigate whether changes in the maternal coagulation profile influence the risk of VTE in AMA and non-AMA women.
References
Holmes VA, Wallace JM. Haemostasis in normal pregnancy: a balancing act? Biochem Soc Trans 2005; 33: 428-32.
Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 2010; 116: 1302-9.
Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011; 118(Suppl 1): 1-203.
Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG 2001; 108: 56-60.
James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006; 194: 1311-5.
Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005; 143: 697-706.
Mallett SV, Cox DJ. Thrombelastography. Br J Anaesth 1992; 69: 307-13.
Sharma SK, Philip J, Wiley J. Thromboelastographic changes in healthy parturients and postpartum women. Anesth Analg 1997; 85: 94-8.
Karlsson O, Sporrong T, Hillarp A, Jeppsson A, Hellgren M. Prospective longitudinal study of thromboelastography and standard hemostatic laboratory tests in healthy women during normal pregnancy. Anesth Analg 2012; 115: 890-8.
Macafee B, Campbell JP, Ashpole K, et al. Reference ranges for thromboelastography (TEG(®)) and traditional coagulation tests in term parturients undergoing caesarean section under spinal anaesthesia*. Anaesthesia 2012; 67: 741-7.
Roeloffzen WW, Kluin-Nelemans HC, Mulder AB, Veeger NJ, Bosman L, de Wolf JT. In normal controls, both age and gender affect coagulability as measured by thrombelastography. Anesth Analg 2010; 110: 987-94.
Ng KF. Changes in thrombelastograph variables associated with aging. Anesth Analg 2004; 99: 449-54.
McCrath DJ, Cerboni E, Frumento RJ, Hirsh AL, Bennett-Guerrero E. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg 2005; 100: 1576-83.
Kashuk JL, Moore EE, Sabel A, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery 2009; 146: 764-72; discussion 772-4.
Wilson D, Cooke EA, McNally MA, Wilson HK, Yeates A, Mollan RA. Changes in coagulability as measured by thrombelastography following surgery for proximal femoral fracture. Injury 2001; 32: 765-70.
Lindqvist P, Dahlback B, Marsal K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol 1999; 94: 595-9.
Jacobsen AF, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium–a register-based case-control study. Am J Obstet Gynecol 2008; 198: 233.e1-7.
Schmidt L, Sobotka T. Bentzen JG, Nyboe Andersen A; ESHRE Reproduction and Society Task Force. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update 2012; 18: 29-43.
Butwick A, Carvalho B. The effect of colloid and crystalloid preloading on thromboelastography prior to cesarean delivery. Can J Anesth 2007; 54: 190-5.
Butwick A, Hilton G, Carvalho B. Non-invasive haemoglobin measurement in patients undergoing elective caesarean section. Br J Anaesth 2012; 108: 271-7.
Nielsen VG, Geary BT, Baird MS. Evaluation of the contribution of platelets to clot strength by thromboelastography in rabbits: the role of tissue factor and cytochalasin D. Anesth Analg 2000; 91: 35-9.
Boyce H, Hume-Smith H, Ng J, Columb MO, Stocks GM. Use of thromboelastography to guide thromboprophylaxis after caesarean section. Int J Obstet Anesth 2011; 20: 213-8.
Butwick A, Ting V, Ralls LA, Harter S, Riley E. The association between thromboelastographic parameters and total estimated blood loss in patients undergoing elective cesarean delivery. Anesth Analg 2011; 112: 1041-7.
James AH. Prevention and management of venous thromboembolism in pregnancy. Am J Med 2007; 120(10 Suppl 2): S26-34.
Ghaji N, Boulet SL, Tepper N, Hooper WC. Trends in venous thromboembolism among pregnancy-related hospitalizations, United States, 1994-2009. Am J Obstet Gynecol 2013; 209: 433.e1-8.
Virkus RA, Lokkegaard EC, Bergholt T, Mogensen U, Langhoff-Roos J, Lidegaard O. Venous thromboembolism in pregnant and puerperal women in Denmark 1995-2005. A national cohort study. Thromb Haemost 2011; 106: 304-9.
Gizzo S, Noventa M, Anis O, et al. Pharmacological anti-thrombotic prophylaxis after elective caesarean delivery in thrombophilia unscreened women: should maternal age have a role in decision making? J Perinat Med 2014; 42: 339-47.
Gorton HJ, Warren ER, Simpson NA, Lyons GR, Columb MO. Thromboelastography identifies sex-related differences in coagulation. Anesth Analg 2000; 91: 1279-81.
Steer PL, Krantz HB. Thromboelastography and Sonoclot analysis in the healthy parturient. J Clin Anesth 1993; 5: 419-24.
Polak F, Kolnikova I, Lips M, Parizek A, Blaha J, Stritesky M. New recommendations for thromboelastography reference ranges for pregnant women. Thromb Res 2011; 128: e14-7.
Boer K, den Hollander IA, Meijers JC, Levi M. Tissue factor-dependent blood coagulation is enhanced following delivery irrespective of the mode of delivery. J Thromb Haemost 2007; 5: 2415-20.
Hellgren M, Blomback M. Studies on blood coagulation and fibrinolysis in pregnancy, during delivery and in the puerperium. I. Normal condition. Gynecol Obstet Invest 1981; 12: 141-54.
Szecsi PB, Jorgensen M, Klajnbard A, Andersen MR, Colov NP, Stender S. Haemostatic reference intervals in pregnancy. Thromb Haemost 2010; 103: 718-27.
Morris JM, Algert CS, Roberts CL. Incidence and risk factors for pulmonary embolism in the postpartum period. J Thromb Haemost 2010; 8: 998-1003.
Tepper NK, Boulet SL, Whiteman MK, et al. Postpartum venous thromboembolism: incidence and risk factors. Obstet Gynecol 2014; 123: 987-96.
Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost 2008; 6: 905-12.
Chan WS. The ‘ART’ of thrombosis: a review of arterial and venous thrombosis in assisted reproductive technology. Curr Opin Obstet Gynecol 2009; 21: 207-18.
Acknowledgement
We gratefully acknowledge Ming Zheng, PhD for statistical advice.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
All authors (Alexander Butwick, Maria C. Gutierrez, Gillian Hilton) made substantial contributions to the conception and design of the study, the acquisition of data or analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content.
Rights and permissions
About this article
Cite this article
Butwick, A., Gutierrez, M.C. & Hilton, G. The impact of advanced maternal age on peripartum thromboelastographic coagulation profiles: a prospective observational exploratory study. Can J Anesth/J Can Anesth 62, 504–512 (2015). https://doi.org/10.1007/s12630-014-0300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0300-0